2020 is definitely a year of review and reflection. ANZGOG has achieved 20 years of conducting clinical trials in Australia and New Zealand and has also had to weather one of the most significant health crisis the world has had to deal with.
Our focus through the later part of the 2019-2020 financial year has been on how we best look after the patients on our clinical trials and ensure that we are able to deliver those trials safely and widely to women with a gynaecological cancer.
COVID-19 represents an unprecedented challenge to the health and research sectors. The safety and well-being of patients, research participants and their families, health care professionals, researchers, and other staff involved in patient care and research have been our priority. We would like to thank our research teams for their contribution to the effective management of all our trials to ensure the safety and wellbeing of the women participating. As a collective, ANZGOG’s response was swift and measured. The outstanding collaboration of our investigators, sites, and operating centres has meant that we were ready to move to re-opening for recruitment as sites came back on line and are confident about the management of our trials looking into 2021.
In 2020 we also passed a significant milestone with 1061 members registered with ANZGOG and the 18 clinical trials underway across our 59 clinical trial hospital sites.
Along with the many members who have been involved in ANZGOG since the beginning in 2000, we are very pleased to see such a large number of trainees, registrars and fellows participating in the organisation. It is also very important to note the role that cancer consumers play in ANZGOG’s research both as patients but also as advisers to our research through our Consumer Research Panel led by Wanda Lawson.
2020 overall has been a good year for ANZGOG’s research portfolio. We have more trials underway than at any prior time. During the year we opened two new trials – STICs and STONEs and IGNITE – and completed recruitment in the MOCCA study. We were delighted with the news that three ANZGOG-led studies were awarded $4.3m in grants by the Medical Research Future Fund - HyNOVA, ADELE and PARAGON-II.
In the last 20 years ANZGOG has enabled 60 studies, including 37 clinical trials, treating close to 4,000 patients. We are very positive about the contribution that ANZGOG and its members can make to the future of gynaecological cancer research both here in Australia and New Zealand and internationally.




















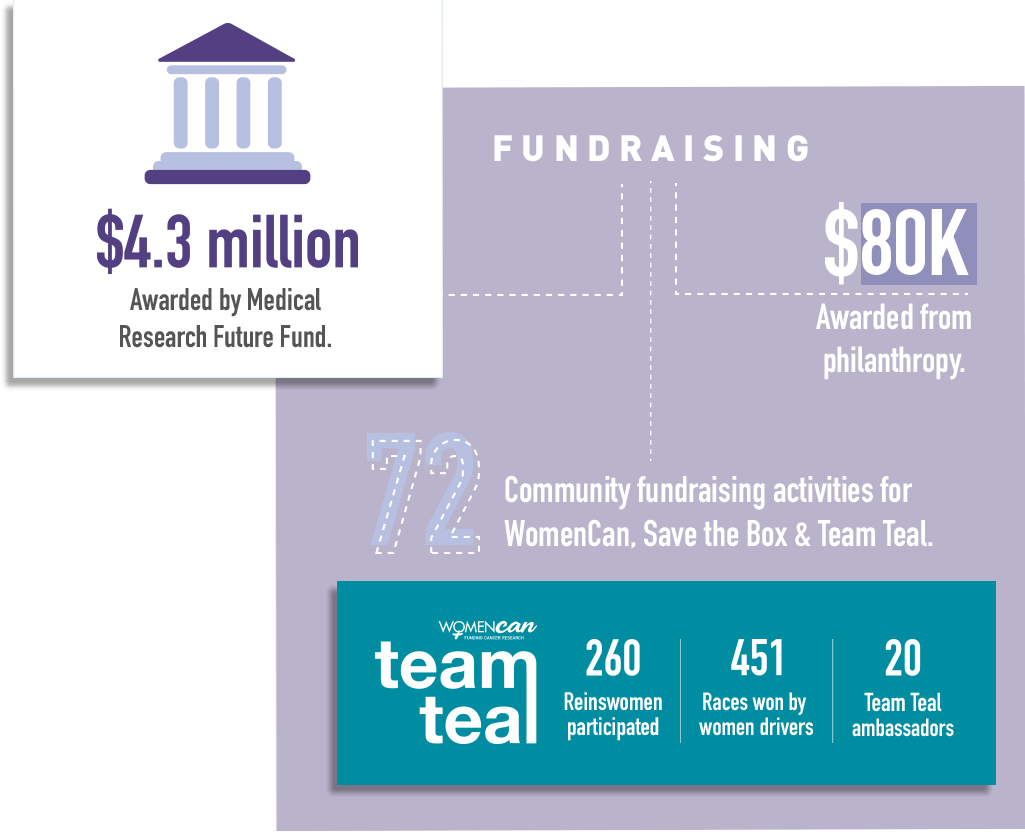
It’s congratulations all round to a number of ANZGOG members for their successful MRFF grant initiatives in reproductive and gynaecological cancers to be conducted under the ANZGOG banner. Prof Linda Mileshkin, Assoc Prof Rhonda Farrell, and Assoc Prof Chee Lee achieved a total of $4.3 million in funding, allocated to projects in the recent MRFF grant announcement via Health Minister Greg Hunt MP.

Endometrial cancer is the most commonly diagnosed gynaecological cancer in Australia. Women with high risk endometrial cancer would typically have surgery followed by chemotherapy and radiation therapy as standard treatment. However, despite having completed this standard treatment, approximately 30% will still unfortunately experience cancer relapse in five years.
The purpose of the ADELE study is to determine if cancer relapse rates can be lowered by adding immunotherapy (tislelizumab) to current standard treatment for high risk endometrial cancer. Quality of life, adverse events and translational research will also be studied. Women will be randomly assigned in 2:1 ratio to either receive standard treatment with or without immunotherapy (tislelizumab).
Patient Recruitment: 268/500
Sites Open: 8/9
Patient Recruitment: 46/110
Sites Open: 18/19
Patient Recruitment: 44/114
Sites Open: 15/15
Patient Recruitment: 10/60
Sites Open: 10/11
Patient Recruitment: 63/75
Sites Open: 10/10
Patient Recruitment: 0/15
Sites Open: 4/7
Patient Recruitment: 1/70
Sites Open: 4/6
Patient Recruitment: 1/60
Sites Open: 4/6
Patient Recruitment: 0/40
Sites Open: 5/7
Patient Recruitment: 7/64
Sites Open: 3/10
The Fund for New Research promotes the development of investigator-led studies from initial concept to full study. In 2019 grant applications were requested for both pre-clinical and pilot or small clinical trials that may lead to a larger and significant clinical trial.
Individual grants are awarded to researchers for amounts of $50,000, $30,000 and $20,000. The Fund for New Research is made possible by public donations, bequests and philanthropic grants from charities with shared interests in ANZGOG’s research work. With this support we have been able to support 14 studies from 2015 to 2019 inclusive.
The following studies were awarded grants for the 2019 round:

Conquer fear from ovarian cancer
Many people living after a diagnosis of cancer experience fears about the cancer coming back or progressing. In some this is the cause of major distress and intrudes into their daily lives. The Psycho-Oncology Cooperative Research Group (POCOG) has previously demonstrated the Conquer Fear psychological intervention is effective in reducing fear of cancer recurrence in cancer in breast, colorectal, and melanoma cancer populations.
In this study we will determine the suitability of this intervention for women diagnosed with ovarian cancer. Using focus groups and interviews we will explore any components of the intervention requiring modification to ensure their suitability for women with ovarian cancer, both women currently disease free and those with advanced disease. This study will enable us to adapt the intervention and assess its place in a stepped-care model to treat fear of cancer recurrence in women with ovarian cancer. It provides the preparatory work to conduct a randomised evaluation of the stepped-care model of Conquer Fear with the ultimate goal of reducing fear of cancer recurrence and progression in women and their partners to improve their daily functioning and quality of life.

Targeting MYC-dependent high-grade serous ovarian cancer via FACT complex inhibition
High-grade serous type ovarian cancer (HGSOC) accounts from most (>80%) cancer death from ovarian cancers and overall survival has not changed over several decades. MYC amplification or activation, linked with tumour aggressiveness, are found in over 50% of HGSOCs. Over several decades considerable effort has been applied to develop drugs to target MYC oncoprotein, with none in routine clinical use. Alternate strategy to target MYC-active tumours is to identify genes that regulates MYC oncogenic activity.
We have identified a gene called SSRP1 that regulates MYC activity in HGSOCs and we have compelling data that CBL-0137, a novel SSRP1 inhibitor, can be repurposed for therapeutic targeting of MYC-active HGSOCs. Our data convincingly demonstrate that CBL-0137 inhibits the MYC pathway and induces cell death in the pre-clinical models of HGSOCs. We will test the effectiveness of CBL-0137 in treating patient-derived HGSOC tumours with MYC pathway activation. We will test this drug in combination with chemotherapy to effectively treat MYC-active chemo-resistant ovarian tumours. The positive outcomes of this work will therefore not only benefit MYC-active HGSOC patients but will have a large impact on any type of cancer patients carrying upregulated MYC oncogenic pathway.

Generating pre-clinical models for uterine leiomyosarcoma to help direct treatment.
Grant provided from funds raised by Rochelle Fisher.
Uterine leiomyosarcoma is a rare form of uterine cancer. Currently, women with this cancer have a 50% chance of being alive 5 years after their initial diagnosis. This dire outcome is largely due to a scarcity of appropriate samples to perform experiments on in the laboratory. Such experiments can tell us what causes the disease and how we can best treat it.
The Stafford Fox Rare Cancer Program based at The Walter and Eliza Hall Institute of Medical Research aims to overcome this by building a ‘biobank’ of uterine leiomyosarcoma tissue. Using this tissue, we can model the disease by growing it in the laboratory and testing different therapy options to see which provides the best response. These experiments will then inform clinicians of the optimal drug treatments for patients with uterine leiomyosarcoma. We can also assess the tumour tissue for different genetic mutations to figure out why the cancer grew in the first place, and which mutations to screen for in order to detect early instances of the disease. Ultimately, we believe that these experiments will lead to better outcomes for uterine leiomyosarcoma patients.

Getting the MOST out of ovarian cancer follow-up.
Grant provided by The Ladybird Foundation for a Western Australia project.
The aims of follow-up of women with ovarian cancer are to manage psychosocial symptoms, late effects of treatment and to detect recurrence. However, there is no evidence to support the current model of clinic-based follow-up of women following chemotherapy for ovarian cancer or how well symptoms are treated. Many patients, particularly those living in rural and remote locations and the elderly, may find it difficult and costly to travel to their hospital clinic appointments.
This pilot study will investigate a novel approach to the follow-up of women with ovarian cancer after completion of surgery and chemotherapy. Patients will have nurse led follow-up with three-monthly video calls and will complete a patient-reported symptom assessment called the Measure of Ovarian Symptoms and Treatment concerns (MOST) questionnaire at home on a personal computer or mobile device, and also have a blood test for the tumour marker CA125. The study aims to show that the intervention is feasible, safe, acceptable and does not delay the diagnosis of recurrence.
Secondary objectives are to assess whether the intervention results in clinical meaningful improvements in emotional wellbeing, health related quality of life, patient satisfaction, the number of patients referred for treatment of symptoms such as anxiety and fear of recurrence, and whether the nurse led follow-up method is more cost-effective than the conventional model. This study will also inform the design of a larger phase III trial.

Targeting metabolism to improve efficacy of ovarian clear cell carcinoma therapeutic agents
Grant provided by the Judith Meschke Bequest
Our goal is to kill ovarian clear cell carcinoma (OCC) by disrupting its sources of energy. We are doing this in two ways:
Our first approach is to disrupt the ability of OCC to use its stores of sugar. Unlike most cancers, OCC stores sugar in the form of glycogen. The glycogen provides an in-built bank of glucose that is consumed instead of external nutrients. In times of stress, including during chemotherapy treatment, the stored glucose is released to provide energy as well as biological building blocks that are essential for the tumours to grow and spread. In this way, OCC can by-pass the effects of chemotherapy by relying on its stored glucose.
We have developed laboratory models using tissue from patient tumours. We have used these to understand how OCCs use energy and we have tested drugs that disrupt these processes. Our results show that at very low doses a drug that blocks the use of stored glucose greatly improves the ability of chemotherapy to kill OCC. In this project we will optimise the use of this drug so that it can then be tested in patients, combining it with chemotherapy.
Our second approach is to develop a thorough understanding of how energy is stored and consumed by OCC. We will do this by comprehensively analysing OCC tumours, using the cutting-edge technique of Mass Spectrometry Imaging, to define those metabolic processes that have the greatest potential to be targeted for the greatest benefit of OCC patients.

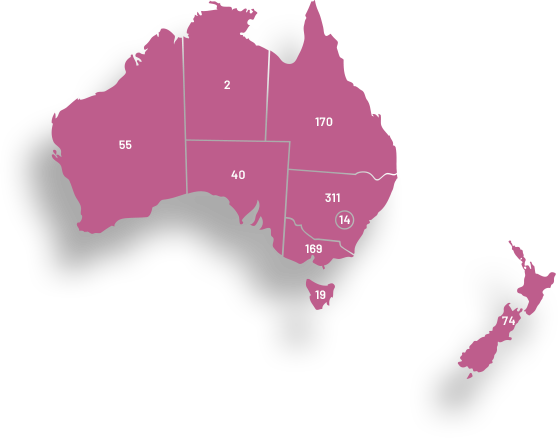
ANZGOG has a diverse membership working at hospitals from every state in Australia and the country of New Zealand. Our members come from a wide range of research, clinical and allied health specialties, demonstrating our collaborative culture and the strength of our outreach into treatment centres for women with a gynaecological cancer.
ANZGOG has more than 1060 members representing clinical, allied health, pure research specialities and women affected by a gynaecological cancer. ANZGOG prides itself on how it operates, with the strength of its collegial and multidisciplinary approach viewed as its core purpose.






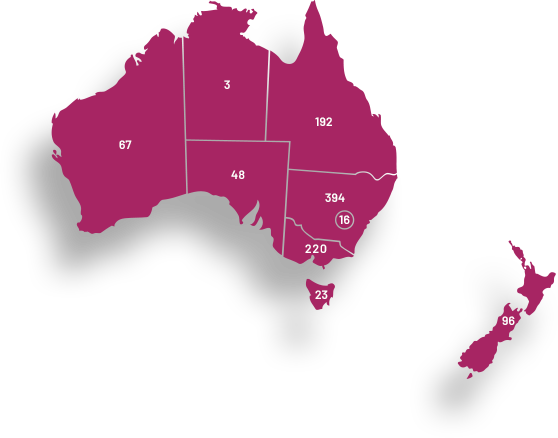
ANZGOG’s clinical trials are conducted at 59 hospital sites in Australia and New Zealand. These trials include both local and global collaborations to ensure the leading research is available for women. We work with metropolitan, regional and remote hospital networks to support their capacity to conduct gynaecological cancer trials.


We actively engage with our members in the Australian and New Zealand clinical research community and our international partners to ensure the relevance, vibrancy and impact of our research agenda. This includes encouraging active participation from the next generation of gynaecological cancer researchers and continuing to foster effective two-way communication with cancer consumers.

ANZGOG’s international collaborations are fostered through its individual members and organisational membership of the GCIG.

Strong local collaborations are fostered with organisations around Australia to develop new trials.

ANZGOG has united with OCA and ASGO to launch the Ovarian Cancer National Action Plan 2020 – 2025.
During COVID-19, ANZGOG’s clinical trials were maintained and recruitment targets were revised in some instances and ANZGOG is on track with the majority of its studies targets despite facing the pandemic challenges. We are grateful to the hard work and support of committees, sites, operating centres, allied health professionals and staff which made this possible.
The 2019 Annual Scientific Meeting was cancelled due to safety concerns with the COVID-19 pandemic. However, ANZGOG has a number of studies in development and negotiation for funding which is ensuring a strong pipeline for 2021 and 2022.

ANZGOG’s international collaborations are fostered through its individual members and organisational membership of the Gynecologic Cancer InterGroup with 32 member countries.
One of the inaugural members, ANZGOG participated in the formation of the Asia Pacific Gynecological Oncology Trials group (APGOT) in November 2019. The group was formed to focus on collaborative studies in gynaecological cancer within the Asia Pacific region, strengthening capabilities and ensuring a greater range of trials available for women.
ANZGOG is already conducting two studies with Singapore as part of the OASIS Research Initiative (VIP and MOCCA).

Strong local collaborations are fostered with institutions such as Sydney and Melbourne Universities, Walter & Eliza Hall, QIMR Berghofer Medical Research Institute , Queensland University of Technology and University of Western Australia are ensuring a diverse approach to research ideas and clinical trial development.
ANZGOG works closely with other cooperative cancer clinical trials groups, the Quality of Life Office, CREST Health Economics and the Genomic Cancer Clinical Trials Initiative to foster new trials and contribute to their development.

ANZGOG has united with OCA and ASGO to launch the Ovarian Cancer National Action Plan 2020 – 2025.
In 2019-2020 ANZGOG united with Ovarian Cancer Australia and the Australian Society of Gynaecological Oncologists to deliver the Ovarian Cancer National Action Plan (NAP) 2020-2025.
The 2020-2025 National Action Plan was launched mid-2020. Its core priorities are:
We are most grateful to all the women with ovarian cancer who contributed to this plan.

Consumer input into research during the 2019-2020 financial year has been wide and varied. We were consumer Associate Investigators on all of ANZGOG’s grant applications during November and December 2019. During the same time period we provided input into TR-ANZGOG - an ANZGOG research initiative that will support the collection of samples from women recruited to ANZGOG trials.
Our input has been both in strategic planning and at the operational level through membership of the patient consent workgroup. We are also well represented on ANZGOG’s Endometrial Cancer Research Initiative through our membership of the QOL/Survivorship working party.
Throughout the year, we have continued our focus on the improvement of lay summaries, participating in a fact-finding workshop on how consumers access health information and working with the team at ANZGOG to improve our lay summaries published in support of ANZGOG trials. Additionally in early 2020, I was appointed as our Consumer Board Member, further demonstrating ANZGOG’s commitment to including its consumers at all levels.
We have an outstanding array of WomenCan, Team Teal and Save the Box community supporters helping us fundraise for research and the conduct of clinical trials at hospitals. These include individual fundraisers, women sharing their stories in the media, on social media and our websites, companies and organisations that run events and fundraisers, thank you all.

Since her Mum’s diagnosis with endometrial cancer in January 2018, Kerri Monteiro has felt compelled to help raise awareness and funds for ANZGOG’s gynaecological cancer research. In 2018 and again in 2019 Kerri organised High Tea events in Adelaide, raising nearly $13,000 to support ANZGOG’s clinical trials.
“Knowing the money that we raised would not only go towards gynaecological cancer research but also helping women in Australia and New Zealand get access to clinical trials when first line treatment didn't work was really important to us and something we wanted to support”.

Alisha Thompson and the Townsville Ovarian Cancer Team hosted a beautiful Teal Afternoon Tea at Riverside Gardens Community Centre on 22nd February 2020 for Ovarian Cancer Awareness Month. It was wonderful to see the Townsville community coming together in memory of Janice Mayes, supported by members of Janice’s family and Mayor of Townsville Jenny Hill.
Everyone had a wonderful day raising over $7,000 for WomenCan. Many thanks to the team, their sponsors, volunteers and supporters. With the Townsville Ovarian Cancer Team having raised over $18,000 so far through various fundraising events, this dynamic team of fundraisers is well on the way to achieving their goal of $50,000.

Debbie Bruce hosted Wagga Wagga's biggest Open Garden & Market Day on 9th November 2019 to help raise vital awareness and funding for gynaecological cancer research.
The inaugural Open Garden & Market Day organised by Debbie and her family included a host of boutique market stalls, live music, a petting zoo for kids, face painting, wine tasting, raffle prizes and local food trucks.
Albury based Medical Oncologist and ANZGOG Member, Dr Christopher Steer was there to support Debbie and made a well-received presentation to the crowd.
Alongside many smiles, the day brought in 700 attendees who helped raise in excess of $20,000.

Duncan McPherson OAM lost his wife Lyn to ovarian cancer in 2010. In failing health, Lyn and her family began fundraising for ovarian cancer research, an initiative that Duncan McPherson connected with his passion for harness racing, founding Team Teal in Victoria that same year.
The WomenCan Team Teal campaign is a partnership with the Harness Racing industry, which expanded to all Australian states in 2017 and to New Zealand in 2018. Across six weeks in February and March each year, all reinswomen race in teal pants to raise awareness and much-needed funds for ovarian cancer research when they secure first across the finish line.
The vision of Team Teal is to continue to expand our efforts in raising awareness and much-needed funds for ovarian cancer research nurses and the Survivors Teaching Students program.

In 2017, when Jane Ludemann was diagnosed with low grade serous ovarian cancer (LGSOC) at 33 years of age, she had never heard of the disease. There wasn't any dedicated research on her subtype of cancer, so she founded a charity (Cure Our Ovarian Cancer) and created partnerships with research organisations around the world. ANZGOG is the chosen recipient of research funding in Australia.
Through the charity, Jane and many other women also diagnosed with LGSOC in Australia have been able to raise vital research funds to invest in dedicated research projects. Together they have fundraised to walk up mountains, shave heads, sell cupcakes and hold trivia nights.
ANZGOG is very proud to partner with Cure Our Ovarian Cancer for LGSOC research.
ANZGOG acknowledges the pharmaceutical support for ANZGOG’s clinical trials:
We’d also like to thank each of the Harness Racing state bodies, Harness Racing New Zealand, Harness Racing Australia and Tabcorp for their ongoing contributions to the WomenCan Team Teal campaign.

ANZGOG acknowledges the following trusts for their funding support; The Derham Green Fund and William G Maxwell Trust/ Percy Baxter Charitable Trust, the Ladybird Foundation, and also Cancer Australia for supporting our Survivors Teaching Students program.


Survivors Teaching Students (STS) is a ground-breaking volunteer program that brings the faces and voices of ovarian cancer survivors and caregivers into the classrooms of health professional students to teach them about women’s experiences with the disease.




ANZGOG’s Survivors Teaching Students teams around Australia have continued sharing their stories virtually (via Zoom), uninterrupted throughout the COVID-19 lockdown, to raise awareness of ovarian cancer and promote the importance of good health communication. The universities we work with hold ANZGOG’s STS program in high regard and did not want the students to miss out on the opportunity to participate in the program.
ANZGOG also held an STS Webinar - My Story Our Journey – in June 2020. In this webinar, we showcased ANZGOG’s STS Program and the value of STS across Australia for students and survivors, educators and clinicians including ANZGOG Director Dr Paul Cohen.

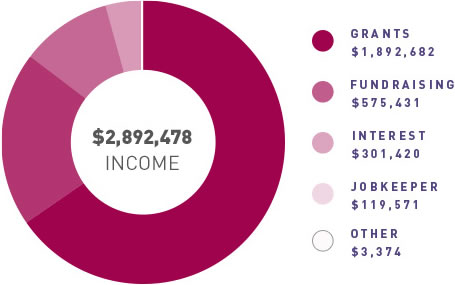
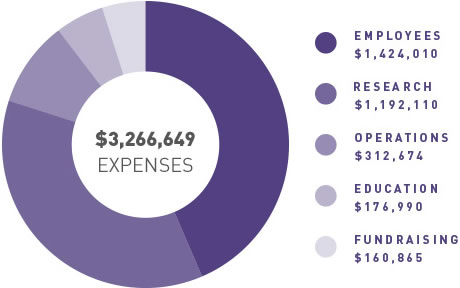
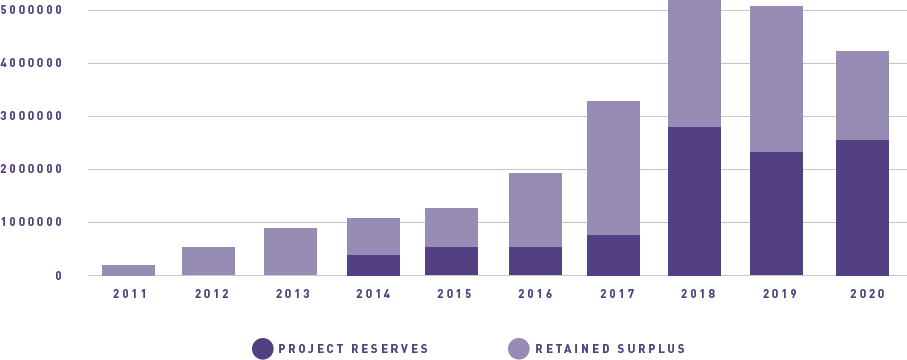
ANZGOG continues to generate funding for clinical trials through grants and pharma support, engage with our many and valued supporters to raise funds through donations and philanthropy, and grow the organisation in a balanced and thoughtful way to support our research agenda.

 View MOre
View MOre